Evidence indicates that large dips in blood pressure at night correlate with progression in normal-tension glaucoma patients.
Carlos G. De Moraes, MD, New York City
2/10/2014
One of the many challenging forms that glaucoma may take is so-called normal-tension glaucoma, in which elevated intraocular pressure appears to play a less-meaningful part in the equation; these patients have IOP measurements within the statistically normal range. Recently, our group pursued a hypothesis regarding the possible connection between this type of glaucoma and blood pressure abnormalities, leading to the discovery of some previously undocumented, clinically useful associations.
Here, I’d like to share some of what we’ve learned so far, and ways in which this may help you care for your normal-tension glaucoma patients.
Systemic Hypertension
Our group became interested in conducting a study after a discussion with a group of internists at Cornell University, led by Mary E. Charlson, MD, William Foley Professor of Medicine and chief of general internal medicine. Dr. Charlson is a renowned clinical epidemiologist who has been studying what happens when patients are treated for systemic hypertension. The literature indicates that the definition of high blood pressure has been slowly changing, lowering the cutoff for what is consider elevated pressure, with the result that patients are sometimes being treated too aggressively. Dr. Charlson’s work is showing that this can have deleterious effects on other organs. For example, patients with very low blood pressure are more likely to have kidney failure, heart problems and even strokes.
Given the fact that glaucoma is a progressive disease with a component of vascular abnormality—especially normal-tension glaucoma—we decided to investigate the possible correlation between glaucoma progression and very low arterial blood pressure, particularly when it occurs during sleep, and in some instances could be due to excessive treatment of systemic hypertension.
Of course, we’re not the first to examine such a connection. However, most recent research on blood pressure and glaucoma has had a different focus than ours. For example, the Early Manifest Glaucoma Trial found a relationship between blood pressure and progression, and some other epidemiologic studies such as the Barbados Eye Study have also suggested this association. However, these studies either relied on a single blood pressure measurement or just checked the pressure during the day, or for 24 hours at most. We know that like IOP, blood pressure varies a lot during the day and from one day to the next. So, we decided to monitor our subjects’ blood pressure every half hour for 48 hours—on three different occasions.
In addition, there’s been no consensus regarding what constitutes low blood pressure, or what parameter should be evaluated to determine whether a patient is at risk or not. So, we not only tested the hypothesis that low blood pressure is associated with progression, we also proposed a method to measure that.
Designing the Study
Our hypothesis was that nocturnal pressure dips could be an additional risk factor for progression, particularly in patients with normal-tension glaucoma, who are known to have a strong IOP-independent component to their disease. The reason that pressure dips are especially dangerous is the phenomenon of autoregulation, which is our bodies’ way of maintaining adequate perfusion in key organs such as the brain and the heart when blood pressure drops. The body narrows the peripheral blood vessels, causing vasoconstriction, reducing the blood flow to less critical organs—including the eye. Of course, the optic nerve is in a watershed zone in terms of circulation, so any decrease in blood flow to the eye can potentially damage the optic nerve. Thus, when blood pressure drops below a key level, the body’s autoregulation kicks in; if the insult persists, we see problems such as hypoperfusion, ischemia and nerve damage.
This problem is exacerbated at night, because blood pressure is normally lower then. Meanwhile, IOP tends to go up at night. Early in the morning, just before you wake up, is when your IOP is normally the highest—at the same time your blood pressure is usually the lowest, causing an imbalance in the blood supply to your eye. Healthy people are able to compensate for this so that it doesn’t cause any damage. But in glaucoma or systemic hypertension, we believe this ability is compromised.
To test whether there is a real association between nocturnal pressure dips and progression, we decided to prospectively monitor patients’ nocturnal blood pressure and see whether those dips correlated with glaucoma progression during the trial period. For our study we selected patients from our office who had normal-tension glaucoma, defined as having all of their untreated IOP measurements below 21 mmHg. They also met criteria such as having at least five visual fields prior to enrollment. Overall, 32 percent of the subjects had been diagnosed with systemic hypertension prior to enrollment and 77 percent of that group were on medications to reduce their blood pressure.
The device we used to measure blood pressure over a 48-hour period was placed on the arm at the same location at which you would normally measure blood pressure; every 30 minutes it automatically inflated and recorded the blood pressure. The device is very noninvasive and reasonably comfortable; I don’t recall any patient ever complaining about wearing it for the 48 hours. (In contrast, checking IOP over a 24-hour period requires waking the patient at intervals through the night, which is not only irritating and disruptive but may also affect the legitimacy of the measurements.) This is one of several such devices that are commercially available, and it’s not very expensive.
After collecting baseline information, including 48-hour blood pressure monitoring, the patients came back at six months and one year for visual field testing and repeat 48-hour blood pressure monitoring.
What the Data Showed
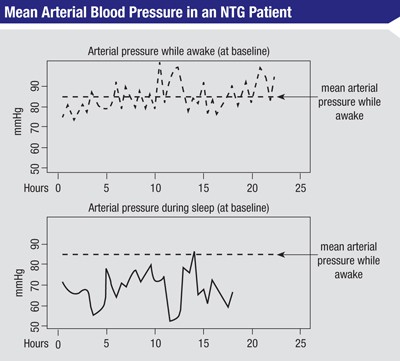
At the end of that year we analyzed the data. From each of the 48-hour measurements, we got an average of the mean arterial pressure during the day when the patient was awake. Then we looked at the blood pressure profile when the patient was asleep and calculated how long the nocturnal mean arterial pressure was below the awake/ diurnal mean arterial pressure, and by what amount (See figure, above).
Comparing this to the rates of progression seen in the visual fields, we found a significant correlation between progression and both the length of time that mean nocturnal blood pressure dropped below the mean diurnal pressure, and the magnitude of the drop. For example, if a patient had a pressure below the mean diurnal arterial pressure for five hours during the night, but he was only a few mmHg below that pressure, he could be at less risk of progression than another patient who dropped below the mean diurnal pressure for only two hours but was 30 or 40 mmHg below the diurnal pressure.
One of our analyses looked to see if patients treated for systemic hyper-tension had a different response than those not being treated. The data showed that for a similar amount of time and magnitude that the nocturnal pressure was below the average diurnal pressure, those being treated for systemic hypertension were more susceptible to progression than those who were not being treated for systemic hypertension. It was clear that people who don’t have hypertension also can experience intense dips, but those who were being treated for systemic hypertension were more susceptible to them.
In short, our prospective study demonstrated that very low blood pressure, particularly at night, is a significant predictor of progression in normal-tension glaucoma patients.
Of course, this does leave an important question unanswered: Is this susceptibility the result of the systemic hypertension, or a side effect of the medications being used to treat the systemic hypertension? To answer that question we’ll need another study, testing the patients after modifying their medications. But at least we’ve shown that there’s an association between being overtreated for systemic hypertension and being more susceptible to the deleterious effects of nocturnal pressure dips.
Red Flags for Clinicians
As ophthalmologists we know that glaucoma with statistically normal pressure has a vascular component. Given the results of our research, we believe that patients with normal-tension glaucoma should be considered for evaluation for nocturnal dips in pressure—and the importance of this testing increases if the patient is being treated for systemic hypertension.
However, there are other red flags besides being treated for hypertension that should possibly trigger such monitoring:
• Postural hypotension. If a patient says that he feels faint when standing up too quickly, that’s an indication that he has low blood pressure in general (i.e., systemic hypotension) and may have a problem with his autoregulatory mechanisms.
• Cold hands and feet. This can indicate insufficient blood flow to the extremities. (If this becomes extreme a person may exhibit Raynaud’s phenomenon, in which extremities have an exaggerated response to cold or emotional stress. Fingers and hands can turn pale, even blue, and become cold to the touch; they may eventually become tingly or numb, and may swell and ache.)
• Migraines. Patients with migraines were very common in our sample—another symptom suggesting an imbalance in the autoregulatory blood pressure mechanism.
• Myopia. Myopia is very common in normal-tension glaucoma patients. At first, many people thought that this association was a data fluke because nearsighted people go to the eye doctor more often, so they were being diagnosed more often. But research, including animal and human research with in vivo imaging, suggests that the optic nerve in myopic eyes is more susceptible to damage because the myopic eye is usually longer, stretching the tissues. Of course, most people with myopia will never have a problem with normal-tension glaucoma, but they are statistically connected. For example, Japan has a higher proportion of myopes than Western countries, and 90 percent of their open-angle glaucomas are normal-tension glaucoma. So if other risk factors such as a genetic predisposition for glaucoma are present, ophthalmologists should scrutinize myopic patients closely.
• Systemic beta blocker use. Plenty of literature has shown that systemic beta blockers—especially used alone—may not be the best way to treat systemic hypertension. One reason is that their main effect is to lower the heart rate and decrease the strength at which the heart pumps blood. When your body senses that blood pressure is dropping, it attempts to compensate by (among other things) increasing your heart rate and the strength of the pumping as part of autoregulation. Beta blockers can interfere with that protective mechanism. Other hypertension medications, in contrast, may act on the vessels or arteries to prevent excessively high blood pressure, allowing the heart to protect key organs when pressure drops at night.
Clinical Management
For screening purposes, ambulatory blood pressure measurements may be performed on patients with normal-tension glaucoma—in particular patients who are progressing despite intensive IOP lowering, for no obvious reason. This will allow you to tell if significant pressure drops are occurring at night. We’ve used the ambulatory device in our office to monitor patients for several years; you just give it to the patient and he comes back 48 hours later. You connect the device to a computer and it shows all the blood pressure information. A clinician can easily use it.
If a normal-tension glaucoma patient is being treated for systemic hypertension, I highly recommend that you talk to the patient’s internist or cardiologist to explain what’s going on and ask whether the treatment might be too aggressive. Does the patient really need such a low pressure? Causing the progression to stop may be just a matter of changing a medication, or having the patient stop taking it at night.
Of course, a few individuals will have this problem at night without having a diagnosis of systemic hypertension. Unfortunately, there are no proven treatments to help in this situation (that we’re aware of), although a clinician could try some unproven treatments that might, in theory, be helpful. For example, salt-loading at night or drinking V8 Juice before bed might help to raise low blood pressure a little during sleep-time. We’ve tried this with moderate success, but again, there’s no clinical evidence to support this approach. It’s based on physiology and our best understanding of what’s happening. Ultimately, we’ll need clinical trials to actually determine whether a given intervention slows or fails to slow glaucoma progression.
Seeing the Whole Picture
It’s important to remember that before being ophthalmologists, we are physicians. We have to look at the patient as a whole. The eye interacts with everything else in the body, and factors such as autoregulation, blood pressure and IOP may be interrelated. Don’t forget to consider other ailments the patient may have, and don’t hesitate to talk to the patient’s internist or cardiologist if blood pressure may be an issue.
The main point I hope clinicians will take from our study is that our suspicions were confirmed: Hypotension during sleep does predict progressive visual field loss in normal-tension glaucoma. So, if you have patients with normal-tension glaucoma—especially those who keep progressing for no apparent reason—it’s very worthwhile to perform ambulatory blood pressure monitoring and see if nocturnal dips are occurring. It’s easy and inexpensive to do, and it may not only explain the reason for the disease and the progression, it might also help you know what to do to prevent future progression. REVIEW
Dr. De Moraes is an associate professor of ophthalmology at New York University Medical Center, and Edith C. Blum Foundation Research Scientist at the New York Eye and Ear Infirmary. He has no financial ties to any product mentioned.


 WhatsApp us
WhatsApp us